
VCE Chemistry SAC 1 Preparation Tips and Tricks
Mar 25
/
Charles Kuang
Why to watch out for SAC 1 of VCE Chemistry Units 3 and 4?
The first VCE Chemistry Unit 3 and 4 sac often takes students by surprise. In general, Year 12 Chemistry is a significant step up to Year 11 Chemistry in terms of difficulty. There are many reasons for this, including but not limited to:
-
Harder exam style questions. In Year 11, the difficulty of most of the questions on your SACs would have ranged from extremely basic to slightly harder textbook questions. However, in Year 12, many of your teachers will take inspiration from exam questions which are longer, have more information, and require a more complex application of the content. If unprepared, you will find yourself struggling to answer questions on your SAC.
-
An accompanying practical. Year 11 sacs are more like topic tests covering certain aspects of the course. In contrast, your first chemistry 3/4 SAC might be based upon a practical, with an additional written component to be done under timed conditions. This adds to the complexity of the assessment, as many of the questions asked will be based upon the practical. Since this is a new situation that will have unique nuisances, you won't get away with memorising answers to common question types alone and will require a much greater level of understanding of the content.
-
More time pressure. One of the biggest challenges in Units 3/4 is managing your time. Depending on the difficulty of your school SAC, expect to struggle finishing it if you don't prepare properly.
What to expect from your first SAC
Your first SAC is likely going to cover a range of topics from fuels and/or redox chemistry.
Here's a potential list of them for you (depending on your school, your sac may cover a combination of these topics):
Here's a potential list of them for you (depending on your school, your sac may cover a combination of these topics):
-
Comparing carbon-based fuels: Fossil fuels vs biofuels, intermolecular forces, comparing physical properties of substances, comparison of fuels, and sustainable chemistry.
-
Energy calculations with fuels: Energic Changes in Reactions, exothermic and endothermic reactions, combustion reactions, thermochemical equations, gases, and calorimetry.
-
Energy from food: Cellular respiration and photosynthesis, proteins, carbohydrates, and fats as a source of Fuel, and the energy content of foods
-
Redox Chemistry: Introduction to redox reactions, oxidation numbers, writing redox reactions, galvanic cells (including commercial examples and primary cells), fuel cells, and producing energy sustainably.
There are a couple common experiments/practical investigations that teachers like to use (this is not an exhaustive list):
-
Determining the energy content of a food/fuel through combustion and specific heat capacity/calorimetry.
-
Investigating the effects of different conditions on the voltage of a galvanic cell.
-
Constructing a fuel cell and measuring its voltage.
-
Investigating energy changes in certain reactions to determine the change in enthalpy of a reaction.
4 simple preparation tips for you to boost your SAC 1 result
1. Learn Your Definitions
Many questions on your first SAC are going to require the use of the following definitions:
-
Fuels
-
Renewable and non-renewable resources
-
Carbon neutrality (with reference to biofuels)
-
Physical properties such as boiling point, melting point, viscosity, cloud point, and flash point.
-
Galvanic cells
-
The role of the salt bridge
-
Primary cells
Remembering (word-for-word) clear definitions that are concise will make answering certain questions on your SACs exponentially faster, eliminating time wasted thinking about how to phrase your answer, and also the hesitation of being unfamiliar with them.
2. Practice the worded questions from VCAA
Your teachers may adapt or even copy some of the worded questions from past VCAA exams, especially longer ones. Even if they don't, answering worded questions from past exams is a great way to train your ability to answer theory/content heavy questions as the skills you learn from answering those questions will be directly transferable to the questions your teacher asks you.
Also, keep in mind that when answering VCAA questions, you should also spend a reasonable amount of time comparing your answer and the answers VCAA gives in the exam report. Only in doing so will you be able to truly reflect on the quality of your responses and make improvements for further questions.
Even if your response meets VCAA's requirements, having a look at the exam report may introduce you to different points and perspectives that you may not have considered before, which can be applied to other questions, in particular, those on your SACs.
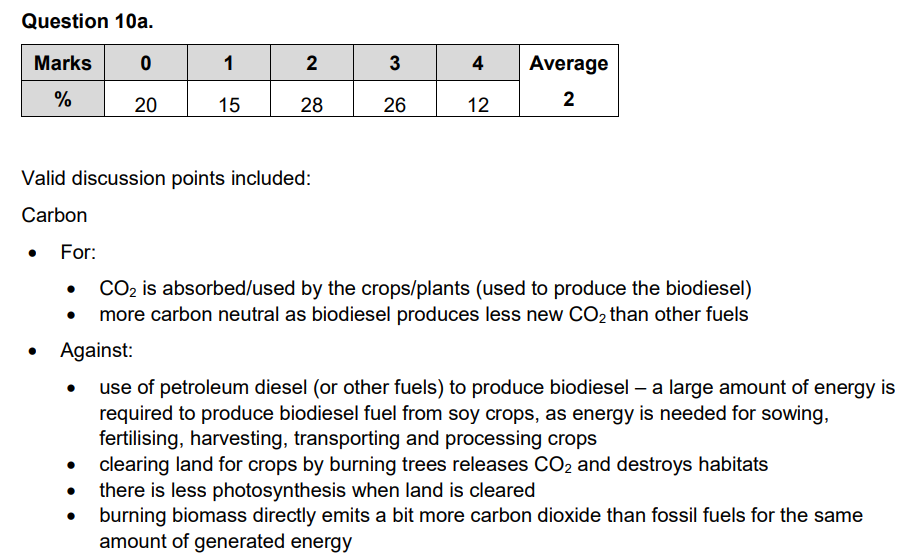
VCAA Chemistry Exam 2019 Question 10a

Model Answer
- Biofuels are carbon neutral because CO2 absorbed by the plants during growth "completely offsets" (quote 3) the CO2 emitted when they are combusted. This leads to a lower net emission of greenhouse gases which is more sustainable for transport as it minimises the contribution to the enhanced greenhouse effect.
- Biofuels are "produced from biological sources such as trees, plants, or microorganisms" (quote 1), meaning it is more renewable than fossil fuels as they can be produced relatively quickly via natural processes. This suggests biofuels are sustainable as fuels for transport.
- However, biofuels are only theoretically carbon neutral as CO2 emissions may arise during production and transportation of the biofuel. Therefore, it may not be as sustainable non-carbon based fuels.
- Also, "dedicating land specifically for generating bioenergy is unwise" (quote 5) as it may compete with agriculture for food. This may exacerbate existing food scarcities or lead to environmental destruction to gain more land for agriculture. This means that biofuels are not entirely sustainable in use as fuel for transport.
Exert of VCAA Exam Report
3. Practice Under Time Conditions
Completing questions without time pressure is important for learning the basics of the content and building accuracy. However, if you neglect to practice under time conditions, you will not be prepared for the stress and anxiety of the actual SAC. Often students will describe performing poorly on their SACs despite knowing the content. The main reason for this is being unable to draw upon their knowledge quickly and efficiently. Therefore, in order for you to make use of your knowledge most effectively is its imperative that you acclimatize yourself to the stress of time pressure.
This means that you should be doing any practice SAC given to you by your teachers or sourced elsewhere under time pressure. Furthermore, when doing practice VCAA questions, you can give yourself 1.5 marks/min as a rough guide (the exam is 120 marks with 180 minutes of reading time). It may not be perfect, but the key is to replicate the anxiety of having a set amount of time to complete questions - it doesn't matter if you estimate the time wrong.
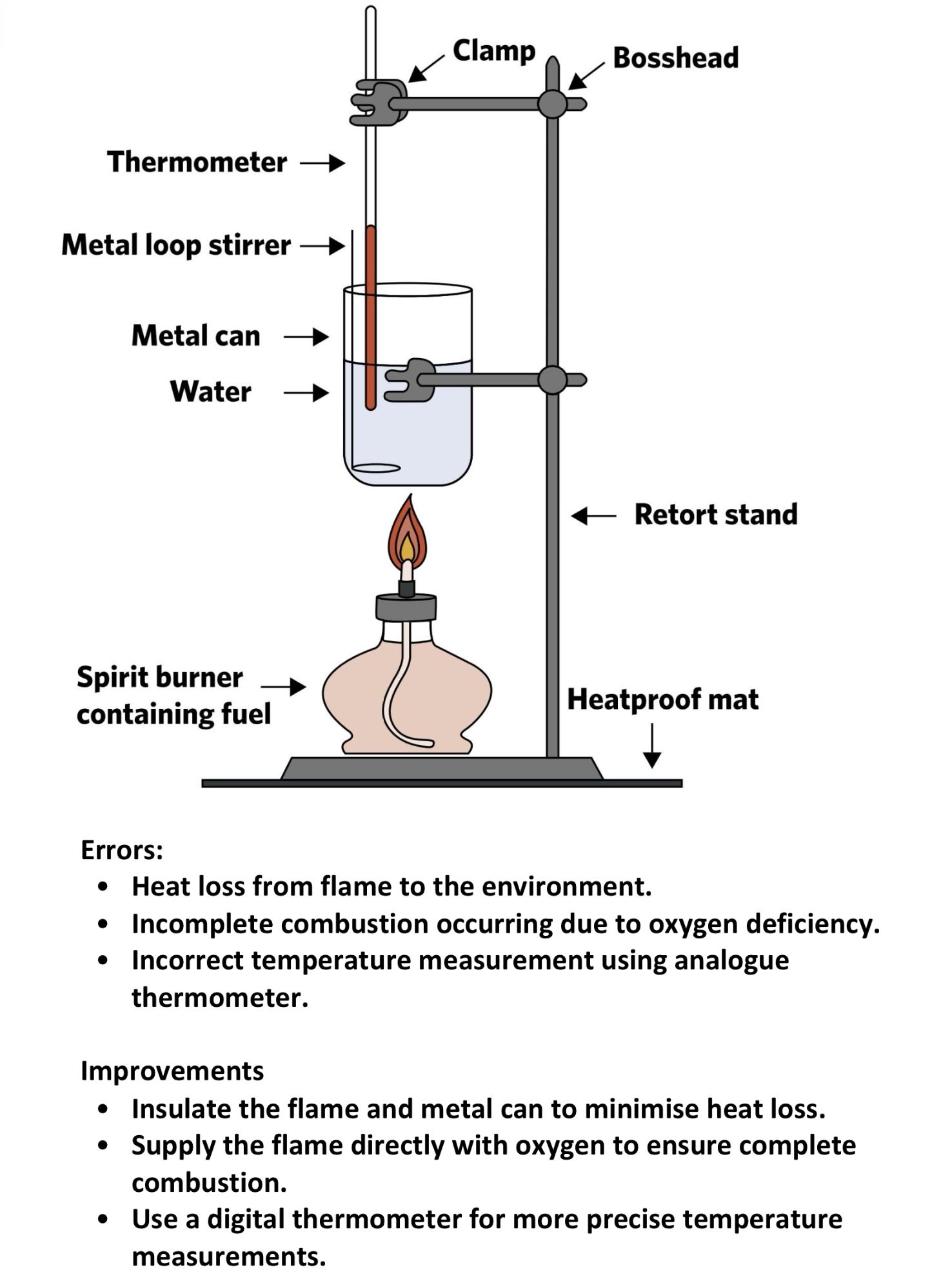
4. Think about errors and improvements in your practical
If your SAC involves a practical, your teacher may ask you to explicitly discuss errors and improvements relating to the practical, or may ask you to write a discussion which will include errors and improvements as well. These can often to be tricky to come up with on the stop and you may end up relying on minor errors/improvements that your teacher may not deem adequate to give marks for. As such, if you brainstorm them in advance, you will have more time to come up with a list of errors and improvements that are impactful and valid, saving you a lot of time and stress during your SAC.
Want to learn more about taking your Chemistry to the next level?
At Artin Education, we believe in supporting students to achieve their full potential. It doesn't matter if you're struggling, or if you're looking to achieve a 50 - if you're interested in maximising your Chemistry score, get in touch now to see what we have to offer!
Featured links
Get in touch
-
contact@artineducation.com.au
-
0430 027 481 (Maths)
-
0490 004 231 (English)
Our Locations
-
Suite 1, 30 - 32 Ellingworth Pde, Box Hill VIC 3128
-
Suite 2, 710 High Street Rd, Glen Waverley VIC 3150
Copyright Artin Education Pty Ltd © 2024